This page provides specifications of knee specimen OKS007. This is a (likely healthy) elderly male specimen candidate.
Specimen Characteristics
Right knee
Gender: Male
Age: 71 years
Race: White
Height: 1.7 m
Weight: 65.77 Kg
BMI: 22.7
Serologically tested: Yes.
X-ray:
Experimentation
-- aerdemir 2015-02-02 14:18:21 All, please complete relevant sections of this page during and after experimentation.
Specimen
Reference Specification: Specifications/Specimens - Revision as of February 1, 2015
Leading Team Member: Snehal Chokhandre
Supporting Team Members: Ahmet Erdemir
Timeline: February 1, 2015 @ ~ 9:30 AM (specimen pulled out of freezer)
Data Location:
Protocol Deviations:
- None.
Notes:
- None.
Specimen Preparation
Reference Specification: Specifications/SpecimenPreparation - Revision as of February 2, 2015
Leading Team Member: Tara Bonner
Supporting Team Members: Snehal Chokhandre, Craig Bennetts
Timeline:
- Thawing: ~9:30 AM February 1, 2015 - ~9:45 AM February 2, 2015
- Dissection: ~9:45 AM - ~10:30 AM February 2, 2015
- Assembly of Optotrak Base Plugs: ~10:30 AM - ~11:00 AM February 2, 2015
- Assembly of Registration Markers: ~ 11:00 AM - ~12:15 PM February 2, 2015
- Probing of Anatomical Landmarks: ~1:30 PM - ~1:45 PM February 2, 2015
- Probing of Registration Markers: ~1:45 PM - ~2:00 PM February 2, 2015
Data Location:
Protocol Deviations:
- None.
Notes:
- Dissected and keeping skin-fat-muscle layers from thigh and shank.
- The tibia Optotrak plug was oriented in an oblique manner (with two screws on the anterior side pointing superiorly).
- The femur registration markers were placed to medial, lateral, and posterior sides (alternative configuration).
- Suture needs to be ordered for upcoming specimens.
- Dissected and keeping the femoral head.
- Dissected and keeping bone from femoral and tibial shafts.
Joint Imaging
Specimen Preparation
Reference Specification: Specifications/SpecimenPreparation - Revision as of February 2, 2015
Leading Team Member: Craig Bennetts
Supporting Team Members: Snehal Chokhandre
Timeline: ~2:00 PM - 2:30 PM February 2, 2015 (patella registration marker will be placed next day)
Data Location:
Protocol Deviations:
- None.
Notes:
- None.
Imaging
Reference Specification: Specifications/ExperimentationAnatomicalImaging - Revision as of February 3, 2015
Leading Team Member: Craig Bennetts
Supporting Team Members: Snehal Chokhandre, Ahmet Erdemir
Timeline:
- Specimen Placement in Transportation Fixture: ~8:30 AM - ~9:00 AM February 3, 2015
- Imaging: ~10:00 AM - ~12:00 PM February 3, 2015
Data Location:
Data was received from the imaging facilities and uploaded to in-house data management server (http://cobicore.lerner.ccf.org/midas; only accessible within Cleveland Clinic network). Raw DICOM files were stored in the folder Open Knee(s) --> Private --> oks007 --> MRI --> DICOM
DICOM files were converted to NIfTI files, stored in the folder Open Knee(s) --> Private --> oks007 --> MRI --> NIFTI of the in-house data management server. NIfTI files were also disseminated to the public in the 'Downloads' section of the project website, https://simtk.org/home/openknee, under the package oks007.
Data storage and dissemination were accomplished based on the updated imaging specifications, see Revision as of April 22, 2015.
Protocol Deviations:
- None.
Notes:
Joint Mechanics
Equipment Preparation
Reference Specification: Specifications/PressureCalibration - Revision as of February 3, 2015
Leading Team Member: Robb Colbrunn
Supporting Team Members: Callan Gillespie
Timeline: ~10:00 AM - ~12:00 PM February 3, 2015
Data Location:
Protocol Deviations:
- None.
Notes:
- Versions of software used during testing
- simVITRO 1.0.3.12
- simVITRO Knee Module 1.0.5.10 - with an exception that the bug introduced in this version with regards to femur CS optimization was manually fixed in the code so that it performed in similar manner to previous and future versions.
- SSCAD Toolkit 1.0.3.18
- SSCAD Toolkit Extensions 1.0.1.3
- SSCAD NDI Optotrak 1.0.0.8
- NDI Optotrak Drivers 1.0.0.6
BioRobotics Software Configuration 1.0.1.5
BioRobotics Common Tools 1.0.6.3
Specimen Preparation
Reference Specification: Specifications/SpecimenPreparation - Revision as of February 3, 2015
Leading Team Member: Tara Bonner
Supporting Team Members: Robb Colbrunn, Callan Gillespie
Timeline:
- Specimen preparation for TFJ testing: ~12:00 PM - ~1:00 PM February 3, 2015
- Specimen preparation for PFJ testing: ~7:30 - ~8:30 AM February 4, 2015
Data Location:
Protocol Deviations:
- None.
Notes:
- None.
Testing
Reference Specification: Specifications/ExperimentationJointMechanics - Revision as of February 3, 2015
Leading Team Member: Tara Bonner
Supporting Team Members:
Timeline:
- Specimen Mounting for TFJ testing: ~1:30 PM February 3, 2015
- Testing of TFJ: ~2:00 PM - ~4:45 PM February 3, 2015
- Specimen Mounting for PFJ testing: ~9 AM February 4, 2015
- Testing of PFJ: ~10:00 AM - ~12:00 PM February 4, 2015
Data Location:
Data was received from the robotics testing facility and uploaded to in-house data management server (http://cobicore.lerner.ccf.org/midas; only accessible within Cleveland Clinic network). Zipped folders were stored in the folder Open Knee(s) --> Private --> oks007 --> JointMechanics under relevant joint related folders.
Zipped folders were extracted and organized in the dissemination folder under Open Knee(s) --> Private --> oks007 --> JointMechanics --> DISSEMINATION of the in-house data management server. DISSEMINATION folder was downloaded and disseminated to the public in the 'Downloads' section of the project website, https://simtk.org/home/openknee, under the package oks007.
Data storage and dissemination were accomplished based on the updated joint mechanics testing specifications, see Revision as of October 21, 2015.
Protocol Deviations:
- None.
Notes:
During tibiofemoral joint testing, 1 Nm internal rotation torque (IE laxity testing at 30 & 90 degrees flexion -- possibly others too) was not captured properly. The IE torque was at 0.5 Nm and off-axis loads (varus) were not minimized to zero. Other levels of loads were fine. A separate trajectory was ran with 1 Nm internal rotation torque starting at 90 degrees flexion down to 0 degrees flexion.
- During patellofemoral joint testing at 0 degrees flexion the sensor did not register much pressure and the location of the pressure sensor needed to be readjusted after the test to accommodate higher flexion angles.
- During patellofemoral joint testing during 30 degrees flexion there was a discontinuity in pressure measurements on the medial side. This may be attributed to sensor wrinkling or cartilage degradation at that area (needs to be checked).
- During patellofemoral joint testing at 60 degrees flexion, 600 N loading was repeated as the clamp cover hose was suspected to catch the fixture edge.
Tissue Mechanics
Specimen Preparation
Reference Specification: Specifications/SpecimenPreparation - Revision as of February 3, 2015
Leading Team Member: Snehal Chokhandre
Supporting Team Members: Craig Bennetts, Ahmet Erdemir
Timeline:
- Soft tissue dissection: ~1:00 PM - ~2:30 PM February 3, 2015
- Cartilage dissection: ~ AM - PM January 4, 2015
Data Location:
Protocol Deviations:
- The team decided to postpone the cartilage dissection to the following day. As a result, bone and cartilage were kept in the fridge another night for dissection in the following morning. All ligament, tendon, menisci segments and secondary tissues were dissected and frozen.
Notes:
- Fat bursa and transverse ligament were obtained as secondary tissues.
Testing
Reference Specification:
Leading Team Member:
Supporting Team Members:
Timeline:
Data Location:
Protocol Deviations:
- None.
Notes:
- None.
Data Analysis
Registration
Spherical Markers
- Femur
<Posterior>oks007_MRG_FMR-P_AGS_01.stl
<Lateral>oks007_MRG_FMR-L_AGS_01.stl
<Medial>oks007_MRG_FMR-M_AGS_01.stl
- Tibia
<Posterior>oks007_MRG_TBR-P_AGS_01.stl
<Lateral>oks007_MRG_TBR-L_AGS_01.stl
<Medial>oks007_MRG_TBR-M_AGS_01.stl
- Patella
<Superior>oks007_MRG_PTR-S_AGS_01.stl
<Lateral>oks007_MRG_PTR-L_AGS_01.stl
<Medial>oks007_MRG_PTR-M_AGS_01.stl
Digitizing Order
- Tibia:
- Sphere 1 = Lateral
- Sphere 2 = Medial
- Sphere 3 = Posterior
- Femur:
- Sphere 1 = Medial
- Sphere 2 = Lateral
- Sphere 3 = Posterior
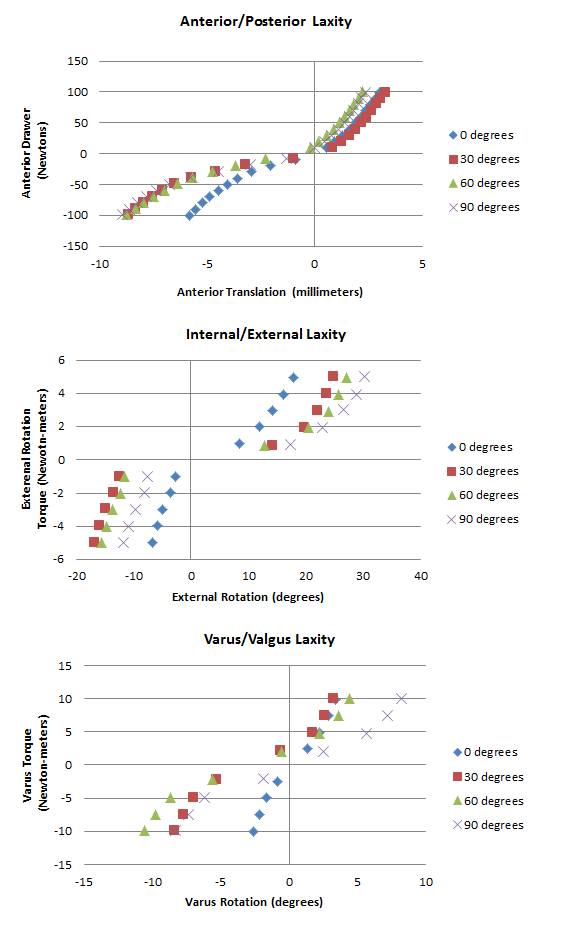
Joint Mechanical Testing
Pictured below are the Anterior Posterior laxity graph, the IR/ER laxity graph, and the Varus/Valgus laxity graph.